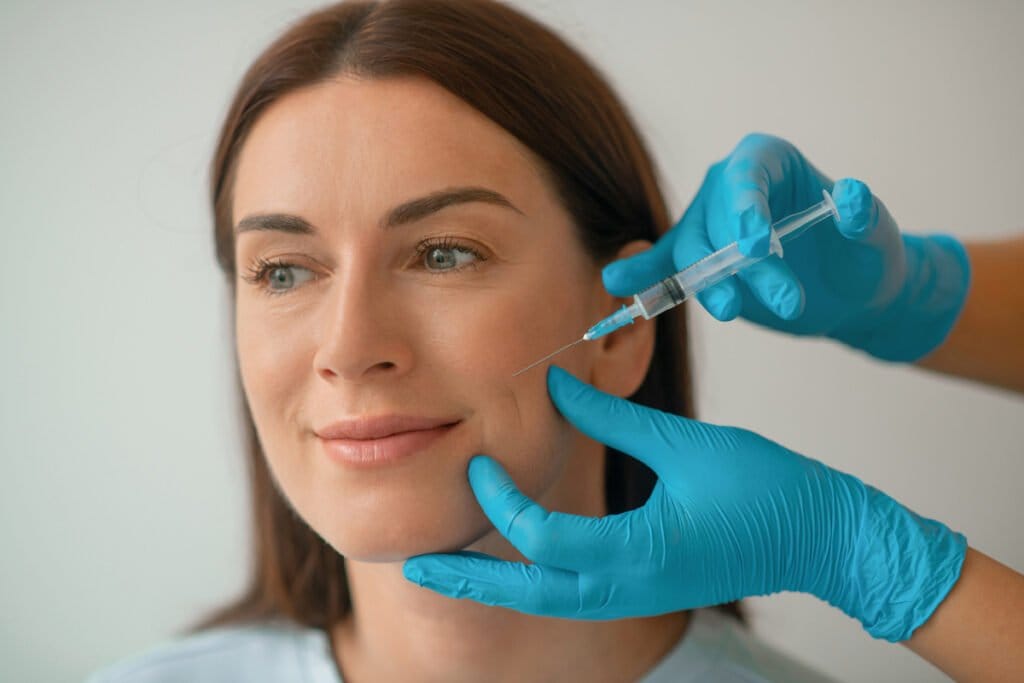
Allergan Aesthetics announced in May that the FDA approved a new dermal filler in the Juvéderm® family of fillers: Skinvive™. While every new injectable promises to achieve somewhat different results, Skinvive is noteworthy because it is injected with a novel microdroplet approach and is indicated to improve skin smoothness, rather than to correct wrinkles or augment features.
The microdroplet technique
As a “microdroplet” facial injectable, Skinvive is injected beneath the skin using numerous very small injection points, spaced about 0.5-1 cm apart, across the treatment area. The filler diffuses within the skin, helping to improve skin smoothness and hydration and to impart an overall glow.
As the goal of Skinvive treatment is to improve skin quality without adding fullness, many are calling this new class of injectable “skin boosters.” While this is a noteworthy departure from the goals of other fillers, it is not entirely new: these injectables are commonly used in other countries, but Skinvive is the first to receive FDA approval in the United States.
Skinvive vs. existing fillers
Facial fillers like Sculptra®, Restylane®, and other Juvéderm products have more cohesive gel formulas and are used to augment and contour features (i.e. cheeks) or fill in deep wrinkles (i.e. nasolabial folds). They are typically injected using techniques that involve inserting the needle and depositing the product while pulling the needle back (linear threading, fanning, and cross-hatching are some examples). This involves depositing a larger amount of filler at a time, with more precision.
Skinvive is the first filler treatment to improve skin radiance, positioning it as an alternative to lasers and other topical skin treatments.
On the other hand, Skinvive has a more water-like texture and is injected in very small “micro droplet” amounts. This allows injectors to treat fine lines, dull skin, volume loss, and mild skin laxity throughout the cheeks by improving the overall skin quality in the area. (Skin boosters are a popular treatment for achieving “glass skin.”) And with Skinvive, patients are unlikely to see an unwanted “pillow face” or over-filled appearance that may result from regular use of filler injections or inexperienced injectors. Depending on your goals, Skinvive may be an alternative to laser skin resurfacing, chemical peels, microneedling, or other medical facial treatments because of its skin-smoothing effects.
Quick facts about Skinvive
- FDA-approved to improve skin smoothness in the cheek area for adults over 21, based on clinical studies
- Safe for all Fitzpatrick skin types I-VI (all skin tones)
- Results last 6-9 months
- Hyaluronic acid (HA) based filler, which works by attracting water to create a more plump, smooth skin appearance (like other Juvéderm dermal fillers)
- Contains a small amount of lidocaine to help with pain during injection
- Mild side effects like bruising, swelling, and redness are common, but most resolve within about 7 days
- Slated to become available at cosmetic surgery practices “within the next six months,” according to a May 2023 press release
Patients should use caution when choosing an injector for any cosmetic medical treatment, vetting the provider for experience, certification, and specific training in facial injectables. Look for a board certified cosmetic surgeon or an injector who works under the purview of one. While nurse and physician assistant injectors may offer injectables, fillers should be prescribed by a physician and authentic name-brand products are only available through a physician’s office or a physician-run medical spa.
Find a cosmetic surgeon near you »